A Forward-Looking Perspective on Mass Tort Practice
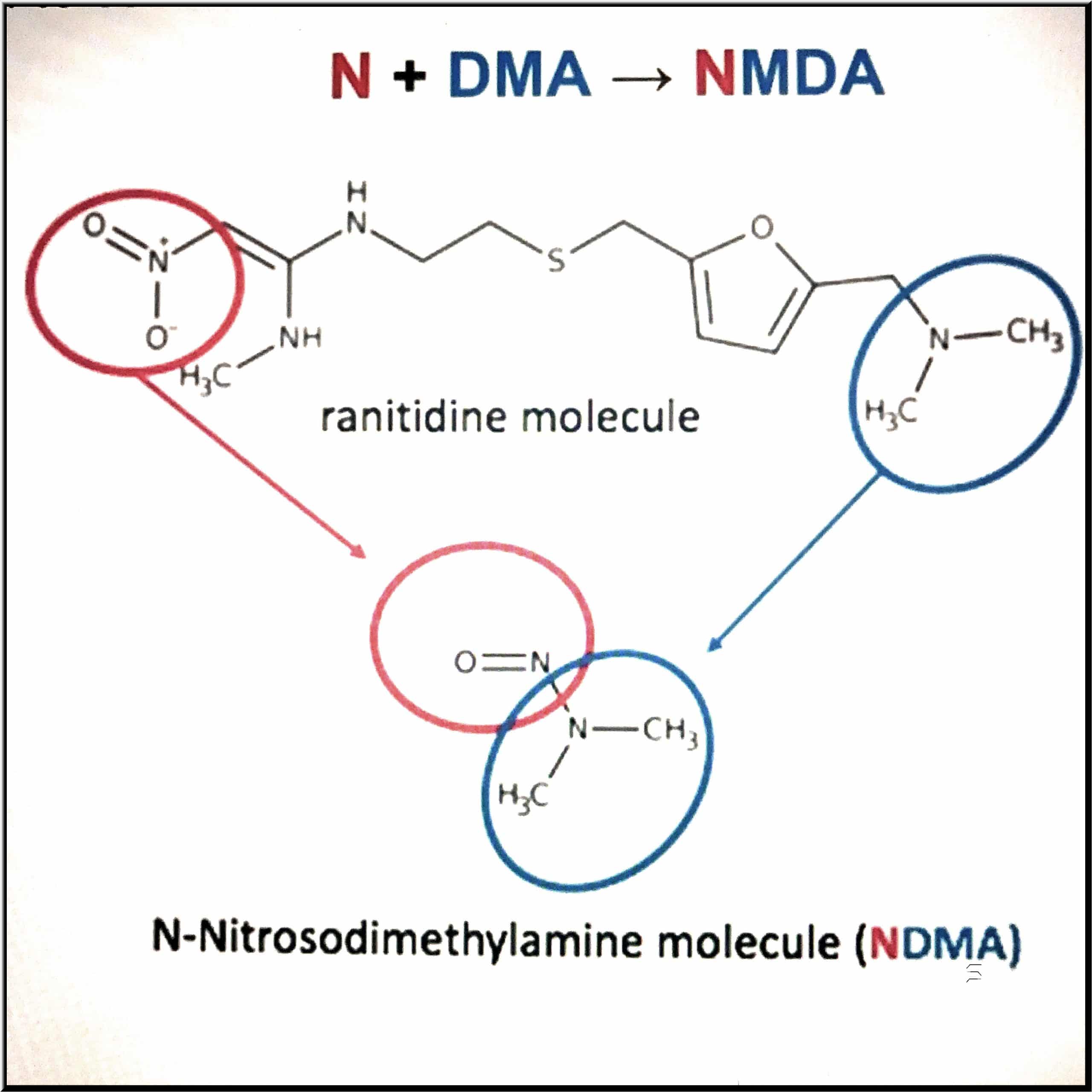
As with most things, the future of mass torts will likely mimic its past. Cancer cases, like those associated with tobacco and asbestos in the past, are driving current and future mass tort litigation. New and ongoing litigation continues for products such as: Zantac Roundup Talc Valsartan Sunscreen PFAS Metformin CPAP With technological and scientific […]